ScienTech Medical reveals impressive one-year follow-up results of ScienCrown® Valve System for TAVR clinical study at GW-ICC2023

On September 8, 2023, at the 34th Great Wall Cardiology Conference titled "The Endless Journey", a presentation highlighting the "One-year clinical study data of ScienCrown® TAVR" was formally opened. The ScienCrown® Valve System uniquely integrates the benefits of both balloon-expandable and self-expanding valves. Its distinctive features, such as its low valve frame design coupled with the incorporation of innovative unlocking technology, guarantee full expansion and complete recyclability. These innovations not only ensure optimal delivery and release stability but also effectively counter prevalent issues associated with TAVR procedures, which includes challenges like suboptimal valve expansion, misalignment, potential displacement, and coronary artery obstruction. Given its design and advantages, the system is particularly suitable for younger patients with aortic valve disorders, which is a solution for the trend of younger TAVR indicated.


A distinguished panel of academic and medical professionals were in attendance at the conference.
The esteemed guests includes Prof. Fang Weiyi, East China Hospital, Fudan University and Prof. Fu Guosheng, Sir Run Run Shaw Hospital, Zhejiang University as the moderator guests.
Prof. Chen Hui, Beijing Friendship Hospital, Capital Medical University, Prof. Han Ke, First Affiliated Hospital, Xi'an Jiaotong University School of Medicine, Prof. Jiang Hong, Renmin Hospital, Wuhan University, Prof. Kong Xiangqing, Jiangsu Provincial People's Hospital, and Prof. Tao Ling, Xijing Hospital, Fourth Military Medical University as the special guests.
Prof. Hu Fudong, First Affiliated Hospital, Zhengzhou University, Prof. Jiang Zhengming, Beijing Anzhen Hospital, Capital Medical University, Prof. Li Jie, Guangdong Provincial People's Hospital, Prof. Lin Xianhe, First Affiliated Hospital, Anhui Medical University, Prof. Ouyang Fan, Zhuzhou Municipal Central Hospital, Prof. Song Guangyuan, Beijing Anzhen Hospital, Capital Medical University, Prof. Wang Yubin, Xuanwu Hospital, Capital Medical University, Prof. Zhang Gangcheng, Zhongnan Hospital, Wuhan University, Prof. Zhang Haibo, Beijing Anzhen Hospital, Capital Medical University, Prof. Zhang Hao, Jiangsu Provincial People's Hospital, and Prof. Zhu Dan, Shanghai Chest Hospital as discussion panel guests.
Prof. Fang Zhenfei, The Second Xiangya Hospital, Central South University, Prof. Luo Jianfang, Guangdong Provincial People's Hospital, Prof. Mei Ju, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Mr. Qiu Kejin, Lepu ScienTech Medical Technology (Shanghai) Co., Ltd., and Prof. Wu Yongjian, Fuwai Hospital, Chinese Academy of Medical Sciences as the academic contributors. (Note: Names have been organized in alphabetical order based on surnames.)
The panel of guests was instrumental in presenting and discussing the latest advancements in the field, contributing to a profound academic discourse during the event.
Opening Address

The progression and utilization of aortic valve intervention valves have consistently remained a central focus of clinical interest. Professor Wu Yongjian, in his inaugural remarks, conveyed that transcatheter aortic valve replacement (TAVR) has assumed a routine role in the field of cardiovascular medicine in China over the preceding decade. During this period, aortic valve intervention valves have witnessed continuous refinement, culminating in the current third-generation aortic valve intervention valves reaching the clinical trial phase. Nevertheless, as TAVR criteria is progressively extended to face a younger and less high-risk patient demographic, the demand for a valve tailored to meet the specific requirements of these younger individuals who characterized by a reduced propensity for coronary artery occlusion and perivalvular leakage, has become increasingly pressing. In response to this need, ScienTech Medical has formally disclosed the the ScienCrown® Valve System, the results from its validation clinical trial supported by a one-year follow-up study at this special conference. In conclusion, we extend our heartfelt wishes for the resounding success of this distinctive conference.
Mr. Qiu Kejin: Design philosophy and clinical application features of ScienCrown

TAVR valves can generally be classified into two primary approaches: self-expanding and balloon-expandable valves, each has distinct attributes. Leading voices in the field advocate for an ideal valve system that synergistically blends the benefits of both these approaches. Driven by this point, eminent structural heart intervention specialists like Professor Wu Yongjian established a collaborative relationship with ScienTech Medical to achieve independent design and development of the ScienCrown® Valve System. In the academic discourse segment of the conference, Mr. Qiu Kejin elucidated on the intricate design intricacies of the ScienCrown® Valve System. He underscored the capability of the system to integrate the dual merits of both balloon-expandable and self-expanding valves. Crucially, this integration proves instrumental in circumventing prevalent complications associated with TAVR procedures. These encompass issues such as suboptimal valve expansion, imprecise positioning, potential displacement, and risks linked to coronary artery occlusion.
Primary design features of the ScienCrown® Valve System:
Precision Structural Design:
1. Short Valve Frame + Self-Expanding: This combination mitigates the risks of pacemaker implantation, perivalvular leakage, and coronary arterial occlusion.
2. Straight cylinder + large opening design ensures effective hemodynamic restoration, ideal for patients with patient-prosthesis mismatch (PPM) risk factors. Its uniform support promotes swift anchoring.
3. Exclusion of implantation at the sinotubular junction (STJ) paves the way for more straightforward reoperations post valve degeneration, which is particularly beneficial for younger patient demographics.
Superior Positioning Functionality:
The system utilizes a full-hook connection design, which enable straightforward valve expansion and recycling. Valve uncoupling is achieved through a dual-step mechanism involving the release and unlock handles of the delivery apparatus. This design ensures that the valve remains functional until uncoupling. Moreover, should the expansion position prove unsatisfactory, the release mechanism can be reversed to attain 100% expansion and recycling.
Innovative Delivery System:
1. The hook system provides gradual release, which maintains an elongated valve form during the release phase.
2. Upon satisfactory valve expansion, the handle of the system allows for unlocking and valve decoupling. The full-hook connection underpins stable uncoupling, obviating potential valve displacement during this phase.
Reasonable Axial Design:
Pre-bending through-the-arch technique: This design facilitates automatic adaptation to the curvature of the aortic arch, which thereby ensure valve crossing coaxiality. Moreover, the capsule assumes a pre-bent orientation during sheath insertion which result in smoother delivery.
Coronary alignment technique: Thanks to the full-hook connection and pre-bent design, the valve adheres to a unidirectional trajectory upon entering the vessel, which seamlessly align with the native annulus post-implantation. This ensures unhindered coronary artery access and mitigates the risk of coronary ostial obstruction.
Coronary alignment technique:
Thanks to the full-hook connection and pre-bent design, the valve adheres to a unidirectional trajectory upon entering the vessel, which seamlessly align with the native annulus post-implantation. This ensures unhindered coronary artery access and mitigates the risk of coronary ostial obstruction.
Versatility in Surgical Approach:
The ScienCrown® Valve System offers clinicians the flexibility to opt between femoral or transapical implantation methods.
Professor Wu Yongjian: Interpretation of one-year clinical follow-up data for ScienCrown®

During the academic discussion session, Professor Wu Yongjian offered an insightful overview of the evolutionary trajectory of the ScienCrown® Valve System, which shed light on the intricate details surrounding the one-year outcomes of the validation trial of the ScienCrown® Valve. Noteworthy is that the ScienCrown® Valve, as underscored by Professor Yongjian, is globally unparalleled—it is the sole self-expanding valve that amalgamates the virtues of a balloon-expandable valve. This avant-garde product, which epitomize a synthesis of medical ingenuity and engineering prowess, encountered several developmental challenges. In the animal testing phase, for instance, thrombus formations were detected. Preliminary analyses linked this issue to the stitching of the valve.
However, the unwavering commitment of the medical engineering research and development team led to a series of innovative methods, one of which involved dual valve implantation in the descending aorta to ascertain thrombi development. Iterative enhancements in valve stitching methodologies triumphed over this thrombus impediment. Further probing divulged that the pivotal breakthrough lay in the valve and direct uncoupling capability of the delivery system. This innovation, which is likened initially to the "rocket separation" seen in satellite launches, epitomized the solution for achieving a minimalistic valve frame design in self-expanding valves. The uncoupling mechanism guaranteed comprehensive expansion and recycling, fortifying the stability and security of the procedure. Equally pivotal, this technology resonated with the shifting paradigms towards younger and lower-risk TAVR beneficiaries. When executed with a long-valve form, it demonstrated unparalleled stability, which ensure unobstructed coronary arteries post-uncoupling. In essence, the trifecta of innovations: the diminutive valve frame design, comprehensive expanding/recycling and the uncoupling feature emphasize the prominent features of the ScienCrown® Valve.
To further substantiate the efficacy and safety of the ScienCrown® Valve, particularly in patients presenting with symptomatic, severe aortic valve stenosis compounded by calcification, an ensuing feasibility trial was embarked upon a 30-day review. Spearheaded by the likes of Professor Wu Yongjian, Professor Fang Weiyi, and Professor Kong Xiangqing, and in concert with principal investigators, an extensive network of 18 clinical trial hubs across China was initiated. This seminal study covered 129 patients diagnosed with severe aortic valve stenosis, deemed unsuitable for traditional surgical valve replacements. Current updates indicate the validation trial of the ScienCrown® Valve nearing completion of its 12-month review, with data collated for 115 patients. Preliminary outcomes are promising—device success at a staggering 98.43%, and procedural success at 99.2%.
At the time of valve implantation, the average maximum transvalvular blood flow velocity reduced dramatically and remained stable in all patients. Within a year of the procedure, the average transvalvular pressure gradient fell significantly and remained at or below 10 mmHg. One-year post-treatment, the average valve orifice area of the patients significantly increased and was maintained at roughly 1.5 cm2 or more. In addition, the average left ventricular ejection fraction of the participants increased dramatically and continued to improve within a year of the surgery. Regarding perivalvular leakage, the vast majority of patients immediately following valve implantation had either no perivalvular leakage or little leakage. In the first year following the treatment, there were no cases of significant perivalvular leakage, and favorable outcomes were routinely maintained. In general, the data corresponds to similar products. (More specific results will be revealed when the late-stage follow-up of the validation trial approaches.)
Professor Luo Jianfang: Application of ScienCrown in high coronary risk Type 1 bicuspid aortic valve

Coronary artery occlusion is a dire complication associated with TAVR procedures. This challenge was astutely addressed by Professor Luo Jianfang, utilizing the ScienCrown® Valve System in a case that entailed severe Type 1 bicuspid aortic valve (BAV) stenosis coupled with a heightened risk to the coronary artery. The patient, a 60-year-old female, manifested a Type 1 BAV characterized by non-fused, calcified leaflets situated on the right, coupled with a depressed left coronary ostium, which produce a pronounced coronary risk. Owing to the risk of coronary obstruction by conventional high stent valve implantations, previous self-expanding valves were constricted to a mere 75% release within the recycling boundary. This constrained release harbored the hazard of incomplete evaluation, spiraling into immediate or subsequent coronary artery blockages. In light of the situation, exhaustive deliberations among the surgical team, which are augmented by a meticulous examination of CT findings, culminated in a collective decision: to employ a 23 mm ScienCrown® Valve for the patient. As the procedure unfolded, an 18 mm balloon was wielded for optimal pre-dilation, subsequent to which the 23 mm ScienCrown® Valve was introduced. Precision was paramount; thus, the positioning of the valve was meticulously tweaked to ensure the marker point resonated with the sinus base. With a methodical release, preliminary angiography portrayed a fully operational valve post-release that was optimally situated. Post-unlocking, the position of the valve remained stable, exemplifying perfect coaxial alignment. Notably absent were regurgitation or perivalvular leakages. A marked drop in the transaortic valve pressure gradient was observed, which plummet from an initial 31 mmHg to a mere 2 mmHg post-procedure. Concurrently, the transaortic valve velocity too decreased from an original 3.86 m/s to 1.2 m/s. The crux of this medical triumph lay in the "short valve frame" architecture of the ScienCrown® Valve, which minimizes encroachments of the coronary artery aperture. Such a design is particularly beneficial for impending PCI and valve replacements so as to bring prolonged benefits for the patient.
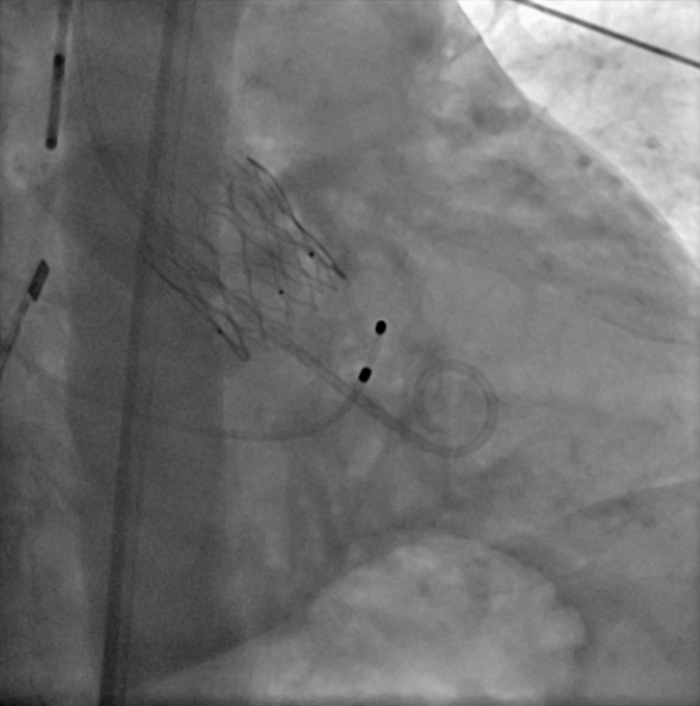
Immediate angiography
Professor Mei Ju: Sharing the case of ScienCrown's alternative “cardiac” approach

Transfemoral (TF) approaches are typically the go-to choice for TAVR procedures. However, clinical complexities arise in cases where patients present with constricted or calcified femoral and iliac arteries, which elevates the risks associated with the TF approach. In these scenarios, the TA approach can serve as an effective alternative, and mitigate potential postoperative vascular complications and the need for pacemaker installations. The ScienCrown® Valve, with its dual models and innovative bidirectional hook design, provides clinicians with the flexibility to opt between a transfemoral or transapical route, contingent on the unique anatomical and clinical circumstances of the patient. It offers a broader spectrum of clinical solutions, especially beneficial for those patients for whom a TF approach might be problematic.
Professor Mei Ju elaborated on this versatility by detailing a particular case involving the ScienCrown® Valve deployed via the TA approach. The patient in that case, a 71-year-old female, underwent CT scans which revealed an aortic valve annulus diameter measuring 20.7 mm. Furthermore, her lower limb vessels demonstrated restricted diameters, which brought challenges for the TF approach by using larger sheaths. Given these findings, the surgical team, after unanimous consensus, opted for a TA implantation of a 23 mm ScienCrown® Valve. During the operation, the TA method was successfully used. Following pre-dilation with an 18 mm balloon, the angiographic readings revealed no waist sign, with only marginal regurgitation. The implantation of the ScienCrown® Valve was slightly challenging. Angiography, prior to unlocking the valve, indicated its less-than-ideal positioning, which led the surgeon to fully expand and recycle the valve twice.
However, the third attempt proved successful, with angiography confirming the optimal positioning and full expansion of the valve. A noteworthy feature of the ScienCrown® Valve is its resilience; despite undergoing full expansions and recycling thrice during the procedure, it retained its structural integrity. These attests of the innovative full-hook connection design ensure stable release processes and the capability for complete expansion and recycling during interventions. Subsequent evaluations conducted immediately post-surgery as well as at intervals of 30 days, 6 months, and 1 year, all revealed positive outcomes. Ultrasound results consistently indicated a well-placed stent, demonstrating even expansion, optimal valve operation, and marginal intravalvular regurgitation. Importantly, there was an absence of any significant perivalvular leakages surrounding the valve frame, and there was no impediment to the coronary arteries.
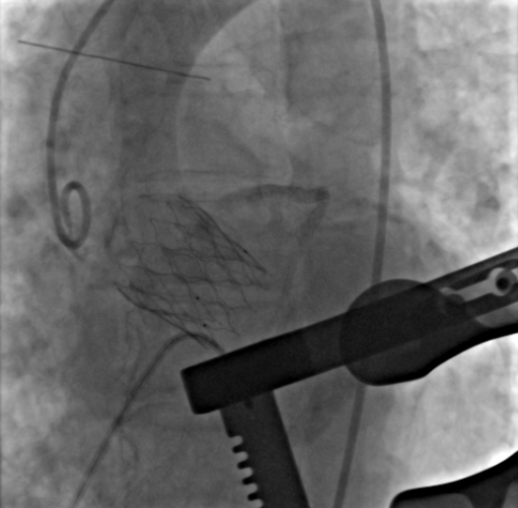
Immediate angiography
Professor Fang Zhenfei: Application of ScienCrown in asymmetrically calcified Type 1 bicuspid aortic valve

In a concluding case study, Professor Fang Zhenfei shared a case of the versatility and robustness by using the ScienCrown® Valve system in handling particularly challenging scenarios. The patient, a 72-year-old male, presented with a severe asymmetric calcified Type 1 BAV, who was characterized by an uneven distribution of calcification. This irregular calcification posed a formidable challenge in crossing the valve. Additionally, the patient had a compensatory hypertrophy of the left ventricle, which, though hypertrophied, was relatively diminutive resulting in decreased cardiac output. Such a condition held the potential risk of circulatory collapse during the surgical intervention. After in-depth deliberations, the surgical team chose to implant a 25 mm ScienCrown® Valve. This particular valve, which is equipped with an array of innovative features such as pre-bending technology in its delivery system, short valve frame self-expanding capabilities, and full-hook connection technology, is meticulously crafted to address intraoperative challenges such as incomplete valve expansion, valve crossing difficulties, and unstable valve positioning. As the surgical procedure progressed, an initial pre-dilation was accomplished using a 22 mm balloon.
Angiography revealed the anticipated asymmetric calcification of the valve annulus, which proved the pre-operative assessment of the potential challenge in delivering the 25 mm valve. However, the ScienCrown® Valve, with its adept pre-bending design, effortlessly navigated through the aortic arch and managed to cross the problematic valve. Subsequent angiographic evaluations confirmed the precise positioning of the valve prior to its unlocking. The culmination of the procedure was marked by angiography that illustrated the artificial valve frame with pronounced echoes situated at the aortic valve location, signifying optimal leaflet functionality and alignment. Notably, there was only a negligible presence of regurgitant flow during the diastole phase of the artificial aortic valve that shows the effectiveness of the valve.
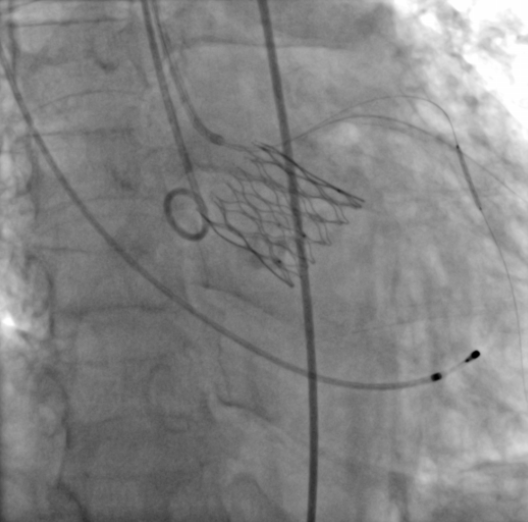
Immediate angiography
Expert Discussion
 Professor Li Jie emphasized the unique short frame of the valve, which facilitates confident high positioning during implantation. The feature of 100% full expansion and recycling is crucial, especially for short self-expanding valves. Additionally, the coronary alignment technology of the ScienCrown® ensures precise alignment of the sinuses during deployment.
Professor Li Jie emphasized the unique short frame of the valve, which facilitates confident high positioning during implantation. The feature of 100% full expansion and recycling is crucial, especially for short self-expanding valves. Additionally, the coronary alignment technology of the ScienCrown® ensures precise alignment of the sinuses during deployment.
 Professor Zhang Haibo expressed initial reservations concerning the potential displacement of the valve. However, personal experiences showcased its superior stability compared to longer self-expanding valves. The design of the valve, especially when deployed via the TA approach, further bolstered its stable positioning.
Professor Zhang Haibo expressed initial reservations concerning the potential displacement of the valve. However, personal experiences showcased its superior stability compared to longer self-expanding valves. The design of the valve, especially when deployed via the TA approach, further bolstered its stable positioning.
 Professor Wang Yubin, having closely worked on the various trials of the valve, commended the stability of the ScienCrown® Valve, even in the absence of a crown.
Professor Wang Yubin, having closely worked on the various trials of the valve, commended the stability of the ScienCrown® Valve, even in the absence of a crown.
 Professor Jiang Zhengming shared initial coaxiality concerns given the release proximity of the valve to the minor curve. Yet, the pre-bending technology of the valve, which adapts to the aortic arch curvature, alleviated these apprehensions.
Professor Jiang Zhengming shared initial coaxiality concerns given the release proximity of the valve to the minor curve. Yet, the pre-bending technology of the valve, which adapts to the aortic arch curvature, alleviated these apprehensions.
 Professor Hu Fudong lauded the versatility of the ScienCrown® Valve, predicting a bright future for its clinical applications due to its dual approach compatibility and full expansion-recycling capabilities.
Professor Hu Fudong lauded the versatility of the ScienCrown® Valve, predicting a bright future for its clinical applications due to its dual approach compatibility and full expansion-recycling capabilities.
 Professor Mei Ju advocated for short valve frames, especially for younger, lower-risk patients, accentuating the edge of the ScienCrown® Valve in preventing coronary artery obstruction due to its unique full expansion and recycling features.
Professor Mei Ju advocated for short valve frames, especially for younger, lower-risk patients, accentuating the edge of the ScienCrown® Valve in preventing coronary artery obstruction due to its unique full expansion and recycling features.
 Professor Lin Xianhe acknowledged the realization of the valve concept envisioned by Professor Wu Yongjian, expressing hope for future aortic valve prostheses advancements inspired by ScienCrown®.
Professor Lin Xianhe acknowledged the realization of the valve concept envisioned by Professor Wu Yongjian, expressing hope for future aortic valve prostheses advancements inspired by ScienCrown®.
 Professor Zhang Hao recounted the benefits of the valve from firsthand experience, especially underscoring its non-intrusiveness in younger patients and the ease of delivery.
Professor Zhang Hao recounted the benefits of the valve from firsthand experience, especially underscoring its non-intrusiveness in younger patients and the ease of delivery.
 Professor Han Ke expressed his deep appreciation for the distinctive features of the ScienCrown® Valve, highlighted during academic discussions by the five experts. He acknowledged the valve's unique charm, combining the benefits of both balloon-expandable and self-expanding valves. This innovative combination provides a new and valuable option for TAVR patients with specific anatomical considerations.
Professor Han Ke expressed his deep appreciation for the distinctive features of the ScienCrown® Valve, highlighted during academic discussions by the five experts. He acknowledged the valve's unique charm, combining the benefits of both balloon-expandable and self-expanding valves. This innovative combination provides a new and valuable option for TAVR patients with specific anatomical considerations.
 Professor Wu Yongjian reflected on the evolution of TAVR and stressed the unique 100% full expanding and recycling capabilities of the ScienCrown® Valve, emphasizing the benefits it brings in terms of post-implantation assessment.
Professor Wu Yongjian reflected on the evolution of TAVR and stressed the unique 100% full expanding and recycling capabilities of the ScienCrown® Valve, emphasizing the benefits it brings in terms of post-implantation assessment.
Summary
The development of the ScienCrown® Valve System is a response to the growing relevance of TAVR indications among younger population. Compared to other aortic valves, the ScienCrown® Valve System represents a significant technological advancement. Professor Kong Xiangqing noted, in conclusion, that the development of prosthetic aortic valves has undergone three phases: implant stability, complication reduction, and recyclability. Formerly, self-expanding valves were considered more stable due to their coronal shape. However, recent mechanical research suggests that artificial aortic valves can be securely implanted by using just the annular area. The results of the one-year validation trial of the ScienCrown® Valve corroborate the results of these mechanical research. Currently, TAVR indications are extending to younger population with aortic valve dysfunction, which place durability at the forefront of aortic valve development. Based on research, shorter valve frames have a longer lifespan than longer valve frames. In conclusion, short valve frames provide both reliable implantation and a longer lifespan. In the future, aortic valves are likely to be designed with short valve frames. Until now, the ScienCrown® valve with its short valve frame appears to be the most promising artificial aortic valve for clinical applications.
-

ScienTech Medical was awarded the second prize of the National Technological Innovation Award
2024-07-03 -

Exhilarating news: ScienTech Medical’s MemoSorb® Biodegradable Patent Foramen Ovale (PFO) Occluder obtained NMPA approval
2023-09-17 -

Good news: Disposable Introducer Sheath obtained Class II Medical Device Registration certificate
2023-06-28
Copyright © 2021, Scientech Medical. All rights reserved. 沪公网安备31011702008238号沪ICP备2021017431号
沪公网安备31011702008238号沪ICP备2021017431号







