Company Profile
As a pioneer specializing in interventional medical devices targeting structural heart diseases and a large China-based supplier of CHD occluders and related procedural accessories, we are committed to providing safe, effective and innovative medical solutions.
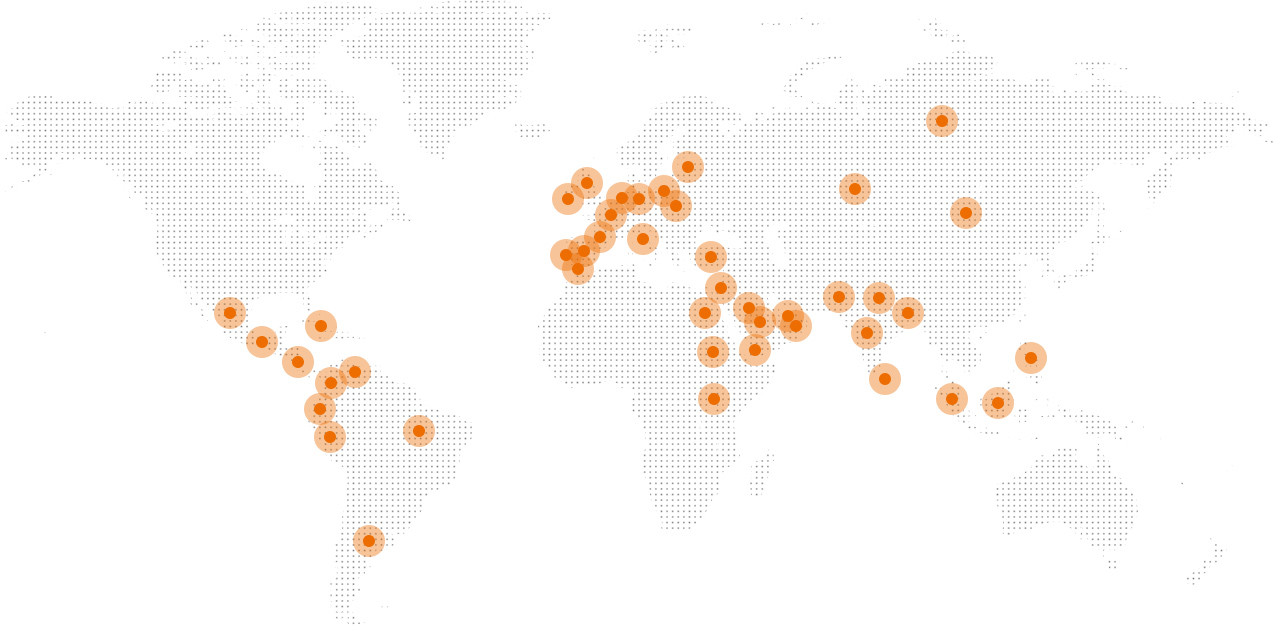
We are engaged in the research and development, manufacturing and marketing of interventional medical devices targeting structural heart diseases. With 50 products available on the market and under development, our product line covers CHD occluders for the interventional treatment of CHDs, LAA occluders and PFO occluders for the prevention and treatment of cardioembolic stroke, and interventional valves; hence, all-round solutions are available for the interventional therapy of structural heart diseases. Our products were sold overseas to 44 countries and regions in Asia, Europe, America and Africa.
We are a strong player in research and development, with a number of innovative products with independent intellectual property rights, taking the lead in the research and development of biodegradable technologies. In 2009, the project of "development and clinical application of interventional treatment series occluder for defective congenital heart" was awarded “Second Prize of National Science and Technology Progress Award". As of April 26, 2023, the Company has been granted 234 registered patents.
We will keep on investing in research and development, and developing novel products and technologies to benefit more patients with structural heart diseases, thereby contributing to the cause of human health.

-
products available on the market and under development
-
patent certifications
-
+
hospitals covered
-
countries and regions where the products are marketed
Corporate Culture
-
Our Mission
Shape better lives with care from “heart”
Social Responsibilities
ScienTech Medical Technology - Far more than care from “heart”: making the warmth of love and healthy companionship accessible to every family
- For patients, we are committed to providing safe, effective and innovative medical solutions
- For doctors and nurses, we live up to every piece of trust and guard each heroic act
- For employees, we guarantee that every ScienTech people can grow with Scientech
- For the society, we are devoted to the public welfare undertakings, and give back to society in different ways
In July 2009, we donated to build the Rehabilitation Center of the Public Health Center of Jiangfeng Town
In 2013, we went to Ali to visit the children with CHDs
In 2017, we donated 30 occluders to Songjiang Women’s Federation and China Children and Teenagers' Fund Songjiang Branch
In 2017, we went to Children's Hospital of Soochow University on the World Heart Day to visit and deliver love to the patients
In April 2022, we together with IPE Center, a subsidiary of Lepu Medical, donated shelter laboratories and equipment with a worth of RMB10 million to Minhang District when the COVID-19 epidemic broke out in Shanghai. Meanwhile, we have guaranteed the supply of antigen detection reagent products to various areas in Songjiang District and Minhang District to ensure the smooth progress of residents' antigen self-testing work
Great undertakings have small beginnings, and difficult tasks are tackled from where it's easy.
ScienTech Medical Technology will keep on striving for the upgrading of human health undertakings.

Milestones
-
In 1994
We were incorporated in Shanghai in May

-
In 2001
Utilizing our VSD occluders, the first "VSD occluder implantation" surgery was successfully operated in China
-
In 2003
Our CHD occluders under the trademark of MemoPart® were approved by the NMPA for commercial use
-
In 2008
We became a wholly-owned subsidiary of Lepu Medical

-
In 2009
Our interventional treatment series occluder for defective congenital heart disease was awarded "Second Prize of National Science and Technology Progress Award"

-
In 2012
Our occluder under the trademark of MemoPart® received CE Mark

-
In 2018
Utilizing our occluders, the first "fully biodegradable occluder implantation"surgery was conducted successfully in China

-
In 2020
Our MemoCarna® ASD Occluder III (Oxide Coating) and MemoLefort® LAA Closure Occluder I were approved by the NMPA for commercial use

-
In 2021
Our MemoCarna® PDA Occluder III (Oxide Coating) was approved by the NMPA for commercial use. Our MemoSorb® PFO Occluder II (Biodegradable), MemoSorb® ASD Occluder IV (Biodegradable) and ScienCrown® Transcatheter aortic valve replacement ("TAVR") system were brought into clinical trials.Guifinder® Blocker conveying system, GuiFlex® NMPA registration approval for integrated interventional delivery sheath tube.

-
In 2022
MemoSorb️®Biodegradable VSD Occluder System and Gruiser® Occluder Interventional Delivery System were approved by the NMPA for commerical use.
ScienTech Medical is officially listed on the main board of the Hong Kong Stock Exchange

-
In 2023
Bio Lefort® First successful implantation of biodegradable left atrial appendage occluder (FIM)
MemoSorb® NMPA registration approval of biodegradable patent foramen ovale occluder
G-cruiser®Biodegradable occluder intervention delivery system, GuiFerry ™ Disposable guide sheath NMPA registration approved
MemoSorb ® Biodegradable atrial septal defect occluder, RF Lance ® Disposable RF atrial septal puncture needle, RF Lance ®Radiofrequency puncture instrument, cross valve guide wire, Ceniper ®The disposable atrial septal puncture system has entered the clinical trial stage
-
In 2024
RF-Lance®radiofrequency puncture generator and RFRF-Lance®disposable radiofrequency atrial septal puncture needle have been approved for NMPA registration.
Ceniper®Disposable Room Diaphragm System NMPA Registration Approval Passed.
MemoSorb® biodegradable atrial septal defect occluder approved for NMPA registration.


We were incorporated in Shanghai in May

Utilizing our VSD occluders, the first "VSD occluder implantation" surgery was successfully operated in China

Our CHD occluders under the trademark of MemoPart® were approved by the NMPA for commercial use

We became a wholly-owned subsidiary of Lepu Medical

Our interventional treatment series occluder for defective congenital heart disease was awarded "Second Prize of National Science and Technology Progress Award"

Our occluder under the trademark of MemoPart® received CE Mark

Utilizing our occluders, the first "fully biodegradable occluder implantation"surgery was conducted successfully in China

Our MemoCarna® ASD Occluder III (Oxide Coating) and MemoLefort® LAA Closure Occluder I were approved by the NMPA for commercial use

Our MemoCarna® PDA Occluder III (Oxide Coating) was approved by the NMPA for commercial use. Our MemoSorb® PFO Occluder II (Biodegradable), MemoSorb® ASD Occluder IV (Biodegradable) and ScienCrown® Transcatheter aortic valve replacement ("TAVR") system were brought into clinical trials.Guifinder® Blocker conveying system, GuiFlex® NMPA registration approval for integrated interventional delivery sheath tube.

MemoSorb️®Biodegradable VSD Occluder System and Gruiser® Occluder Interventional Delivery System were approved by the NMPA for commerical use.
ScienTech Medical is officially listed on the main board of the Hong Kong Stock Exchange

Bio Lefort® First successful implantation of biodegradable left atrial appendage occluder (FIM)
MemoSorb® NMPA registration approval of biodegradable patent foramen ovale occluder
G-cruiser®Biodegradable occluder intervention delivery system, GuiFerry ™ Disposable guide sheath NMPA registration approved
MemoSorb ® Biodegradable atrial septal defect occluder, RF Lance ® Disposable RF atrial septal puncture needle, RF Lance ®Radiofrequency puncture instrument, cross valve guide wire, Ceniper ®The disposable atrial septal puncture system has entered the clinical trial stage

RF-Lance®radiofrequency puncture generator and RFRF-Lance®disposable radiofrequency atrial septal puncture needle have been approved for NMPA registration.
Ceniper®Disposable Room Diaphragm System NMPA Registration Approval Passed.
MemoSorb® biodegradable atrial septal defect occluder approved for NMPA registration.
Worldwide Footprint
With products exported to Brazil, Italy, Russia and other countries and regions, we have established a three-tier quality control system based on domestic medical device laws and regulations, EU medical device laws and regulations as well as the relevant international quality authentication standard.
Copyright © 2021, Scientech Medical. All rights reserved. 沪公网安备31011702008238号沪ICP备2021017431号
沪公网安备31011702008238号沪ICP备2021017431号








