World’s first implantation—Professor Pan Xiangbin’s team, Fuwai Hospital, Chinese Academy of Medical Sciences (CAMS) successfully implanted the MemoSorb® fully biodegradable occluder following its launch
* This article is a reprint of an article published by Fuwai Hospital, Chinese Academy of Medical Sciences
Recently, Pr. Pan and his team from the Center for Structural Heart Disease, Fuwai Hospital(CAMS) successfully completed the world’s first implantation of the MemoSorb® fully biodegradable occluder following its launch in China via the novel TEE guidance operation. This success not only marks the beginning of the global clinical application of the MemoSorb® fully biodegradable occluder, but also a new stage for interventional treatment of structural congenital heart disease in China.
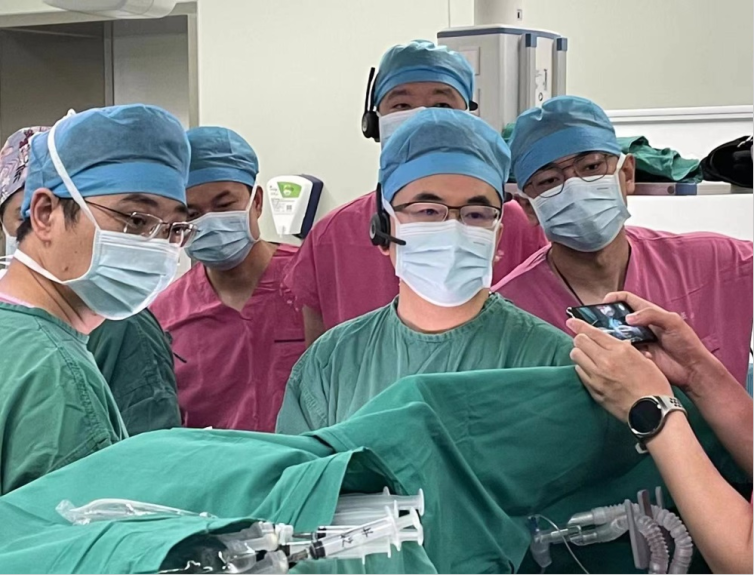
PART 1
First victory—MemoSorb® fully biodegradable occluder successfully implanted
Case Introduction
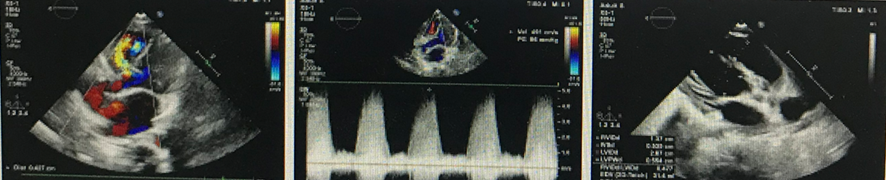
The patient was a 3-year-old boy, 100cm in height and 15kg in weight. The endocardiography on admission showed: an echo loss of about 4mm on perimembranous IVS, continuous normal IAS, IVS thickness of about 6mm, EF 78%; a left-to-right systolic shunt at the ventricular level with high-speed signal, peak flow velocity at about 4.9 m/s;, TDI of about 15 cm/s of the tricuspid annulus. The patient then was diagnosed “pmVSD, and left-to-right shunt at the ventricular level”.
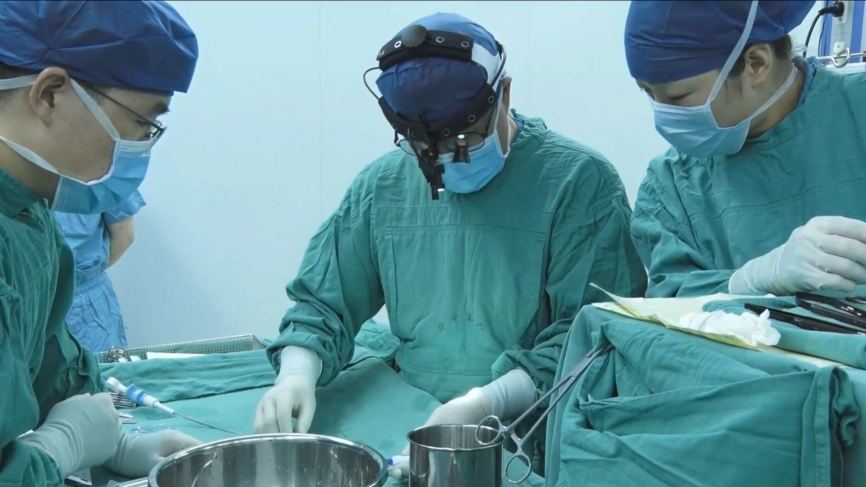
Operation Procedural
This was TTE-guided transthoracic VSD occlusion. First, made a 2cm subxiphoid incision form epidermis to muscle tissue layer by layer, partially separated the sternum, incised and suspended the pericardium to expose the right ventricular free wall. Administered heparin(100 U/kg) intravenously. Pre-operation TTE indicated a tunnel-shaped VSD with the LV surface of 5.6mm and the RV one of 4mm. So the team decided to use a fully biodegradable occluder with a waist diameter of 6 mm. Touched the avascular area on RV surface with finger pulp, found the site directly opposite the defect and chose the puncture site. Use a 5-0 Prolene line with gasket to purse-string suture, inserted a 16G puncture needle into the right ventricle. Inserted the guidewire into the right ventricle with the puncture needle. After confirming that the guidewire entered the left ventricle via VSD, the puncture needle was removed.
Use TTE to guide the delivery catheter to place along the guidewire into the left ventricle, then removed the guidewire. The fully biodegradable occluder was implanted via the delivery sheath for occlusion, and the tug test was performed before releasing. After TTE checking there was no residual shunt or MV incompetence and the occluder was in good morphology and position, released the occluder. The shaping line was cut off and drawn out from one end. The shape locking design is a patented design of Shanghai Shape Memory Alloy Materials Company(SHSMA), which aid in better shaping of the occluder made of degradable materials. In addition, the shaping line can be used as a safety line: if the occluder dislodges during the operation, operator can use shaping line to retrieve the occluder. Removed the delivery catheter, did hemostasis for the puncture wound. Place routinely the pericardial drainage catheter, and sutured the incision to close the chest.

MemoSorb® Fully Biodegradable Occluder In Vitro
PART 2
“Green treatment” of CHD + Implantation without residue
Lead by Academician Hu Shengshou(the Center for Structural Heart Disease, Fuwai Hospital, CAMS), Pr. Pan Xiangbin and his team have been working for decades to put TTE-guide intervention technology into clinical application, and formed a methodology that not only expands the indications of traditional interventional treatment, but also mitigates the negative effect of radiation and contrast, which providing a better choice for CHD gravidus, patients with renal insufficiency or allergic to contrast agents. Besides, they bring out new device, new technique and novel ideas to the intervention of cardiovascular diseases. The MemoSorb® fully biodegradable occluder is a typical example of advancing in device development based on TTE-guided intervention technology.
Currently used CHD occluders are all mainly made with nitinol framework, after implanted in children, those metal remains in the heart for life time and may causes heart abrasion, conduction block, stress imbalance or heart growth problems. In contrast, the MemoSorb® fully biodegradable occluder gradually degrades after occlusion complete, leaving no foreign matter in body. This is a landmark in structural heart disease interventional treatment.
PART 3 Original technology + Original device
China leads the world in CHD diagnosis and treatment!
The combination of TTE guidance and the novel occluder has not only expanded the indications of traditional interventional treatment for cardiovascular disease and realized the treatment of structural heart disease with “no surgery, no radiation, no contrast, and no general anesthesia”, but has also further helped patients avoid metal occluder-related complications as “implantation without residue” saying. This has brought about significant benefits to patients and realized “green treatment” for CHD. At present, TTE-guided percutaneous intervention technology is attracting many centers with its tremendous advantages. It is believed that the promotion of this technology will help patients in China enjoy more minimally invasive and safer treatment; also that with expanded clinical application of MemoSorb® fully biodegradable occluder, the CHD development in China will move to a new stage, more patients will benefit to the greatest extent from the joint application of “original technology and original device!”
Submitted by: Center for Structural Heart Disease Verified by: Pan Xiangbin, Director of the Center for Structural Heart Disease
- Last NewsThe 3-year follow-up of a fully biodegradable implantable device closure for perimembranous ventricular septal defects in children using echocardiography
- Next News First batch of implantation—Professor Fan Taibing’s team successfully apply the MemoSorb® fully biodegradable occluder in two cases of interventional subaxillary pathway VSD occlusion
-

The 3-year follow-up of a fully biodegradable implantable device closure for perimembranous ventricular septal defects in children using echocardiography
2024-07-03 -
First batch of implantation—Professor Fan Taibing’s team successfully apply the MemoSorb® fully biodegradable occluder in two cases of interventional subaxillary pathway VSD occlusion
2023-01-29 -

用于先天性心脏病治疗的全球首款全降解封堵器系统获国家药监局批准上市
2022-02-24
Copyright © 2021, Scientech Medical. All rights reserved. 沪公网安备31011702008238号沪ICP备2021017431号
沪公网安备31011702008238号沪ICP备2021017431号







