First batch of implantation—Professor Fan Taibing’s team successfully apply the MemoSorb® fully biodegradable occluder in two cases of interventional subaxillary pathway VSD occlusion
*This article is a reprint of an article published in the journal Clinic
“The invention of fully biodegradable occluder is a revolutionary process in congenital heart disease (CHD) and the new ceiling in the interventional field of CHD!”
—Professor Fan Taibing
The MemoSorb fully biodegradable occluder embodies the concept of “intervention without implantation” in the treatment of ventricular septal defect (VSD) occlusion and has attracted the attention of clinicians following its successful NMPA approval. On July 26, 2022, Professor Fan Taibing from Children’s Heart Center, Fuwai Central China Cardiovascular Hospital successfully led his team to complete subaxillary minimally invasive VSD interventional treatment for two children with VSD, becoming the first batch in China to use the MemoSorb fully biodegradable occluder. These cases again verifies the safety and feasibility of the MemoSorb in minimally invasive occlusion interventional treatment.
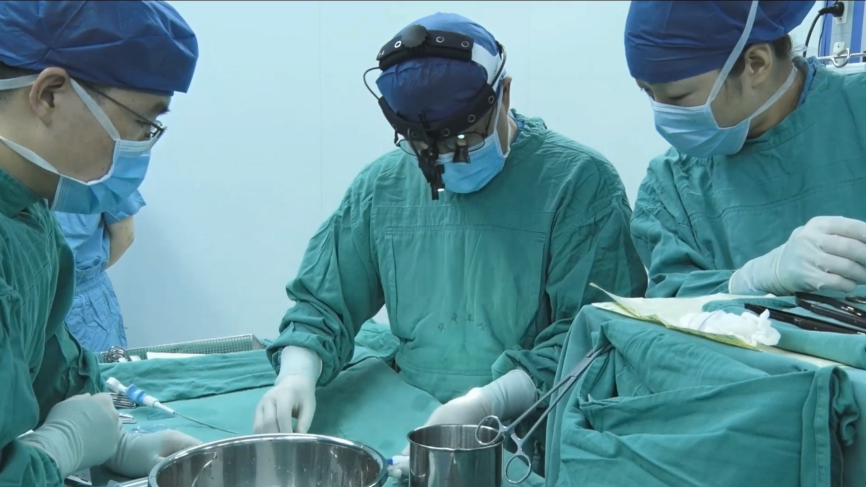
A series of victories—Small incision of subaxillary pathway VSD intervention
MemoSorb fully biodegradable occluder system
The first case is a 3-year-old boy, echocardiography showed “a VSD of 5.5 mm with left-to-right shunt at the ventricular level, continuous atrial septum, no patent ductus arteriosus. Valves with smooth echo and normal movement. Aorta and its branches inner diameter widened. Left heart enlarged, Right heart internal dimension normal. Ventricular septum and LV wall thickness normal, normal movement”. Based on the family opinions of the patient child, as well as medical team discussion, Pr. Fan decided to perform a VSDO using our self-developed MemoSorb fully biodegradable occluder.

Preoperative Echocardiography
Guided by TTE, a right subaxillary small incision pathway was performed. After general anesthesia, TTE examination was taken to determine the size and position of the occluder, and its adjacent structure. Routine heparin was administered intravenously. Operator make a 2-cm right subaxillary small incision, entered the thoracic cavity from the 4th intercostal space, and punctured through the RA free wall. A guidewire was inserted from the TV through the VSD to the LV chamber to establish a delivery track. Then, the fully biodegradable occluder with a waist diameter of 6 mm was placed through the delivery sheath. The occluder was released after TTE confirmed that there was no residual shunt, the valve opening and closing function was normal, and the occluder was in a good position. The shaping line was cut and pulled out with one end, and the catheter was removed and the incision sutured. The operation was successfully completed.

Postoperative Echocardiography
Subsequently, Pr. Fan and his team successfully completed the subaxillary minimally invasive VSD interventional treatment for another two-year-old child with VSD using the MemoSorb fully biodegradable occluder. The operation was successful and the postoperative echocardiography suggested an ideal occlusion with no influence on valve movement.
Expert interviews—Fully biodegradable occluder VSD treatment
“Intervention without implant” and elaborate design reduced the possibility of future heart blocks in patients
MemoSorb fully biodegradable occluder
Professor Fan Taibing: For the two operations today, we both used subaxilliary small incision under TTE-dependent guidance, and achieved good morphology of occluders, ideal closure with no PDL or valve movement dysfunction. Compared with the very first implantation of MemoSorb, we have a deeper understanding and more surgical experience of this fully biodegradable occluder.
According to the previous multicenter clinical research carried out across the country, results of the premarket clinical trial(more than 100 cases) and a two-year follow up, the safety and excellent therapeutic effect of the MemoSorb fully biodegradable occluder have been confirmed. In clinical practice, the pusher feed in the delivery system and the shape locking design of the occluder provide greater convenience for clinical surgeons. The occluder is made of a biodegradable material that is soft and therefore prevents damages to the heart. Due to the differences in materials and design between the MemoSorb fully biodegradable occluder and the conventional occluder, surgeons using the former need to follow more precise operations. For example, the surgeon must accurately select the size of the occluder, and accurately and smoothly complete the release and shaping of the occluder, to avoid device damage caused by repeated recovery. Therefore, the surgeon must have a deep understanding of the device before use, to achieve a better clinical effect.
-

The 3-year follow-up of a fully biodegradable implantable device closure for perimembranous ventricular septal defects in children using echocardiography
2024-07-03 -
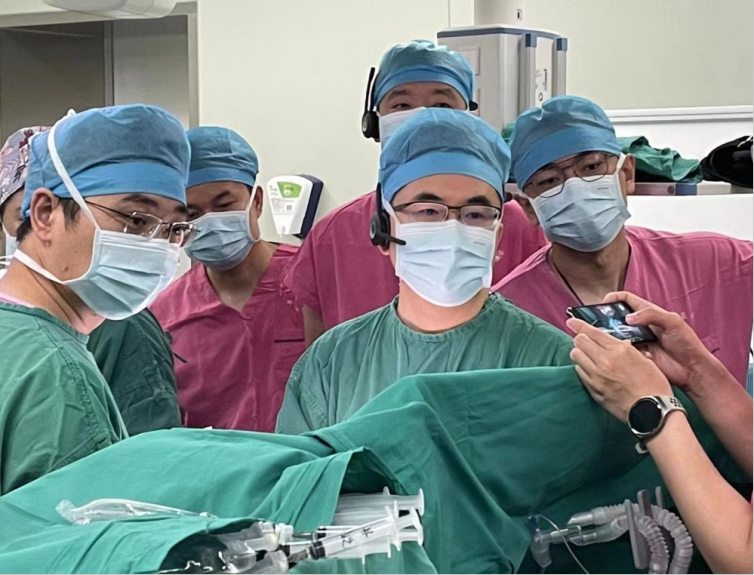
World’s first implantation—Professor Pan Xiangbin’s team, Fuwai Hospital, Chinese Academy of Medical Sciences (CAMS) successfully implanted the MemoSorb® fully biodegradable occluder following its launch
2023-01-30 -

用于先天性心脏病治疗的全球首款全降解封堵器系统获国家药监局批准上市
2022-02-24
Copyright © 2021, Scientech Medical. All rights reserved. 沪公网安备31011702008238号沪ICP备2021017431号
沪公网安备31011702008238号沪ICP备2021017431号







