
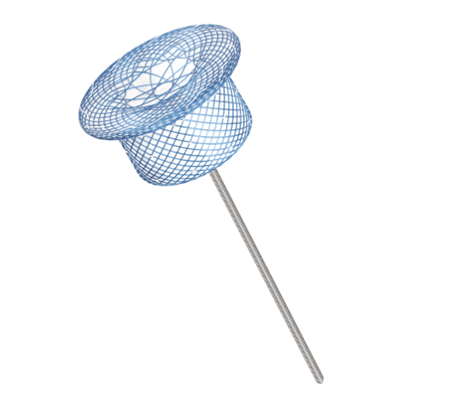
PDA Occulder (Oxide Coating)
Medical device registration certificate No.: GXZZ 20213130335
Description
For treatment of CHD PDA.
Features
Single-hub design: The left disc hub is replaced by the datura-shaped braided mesh for allowing more flattened discs to facilitate endothelialization.
The cylinder or cone shape ensures a close and stable fit to the wall of ductus arteriosus.
With plasma treatment process, a dense and uniform oxide layer is formed on the surface of wire to minimize the release of nickel ions.
Models & Specifications
| DMWBFDQ-I | 04-22 |
| DMWBFDQ-II | 04-22 |
Registration Certificate No.
GXZZ20213130335
(Please consult your local dealer for details)
e-IFU
|
Product Name
|
IFU Revision / Issue Date
|
IFU Language
|
Implant Card
|
|
MemoCarna® PDA Occluder
|
Rev.01
|
EN (Click to download)
|
EN (Click to download)
|
|
Issued on: /
|
Copyright © 2021, Scientech Medical. All rights reserved. 沪公网安备31011702008238号沪ICP备2021017431号
沪公网安备31011702008238号沪ICP备2021017431号







