Description
For transcatheter, percutaneous closure of congenital patent ductus arteriosus (PDA).
Features
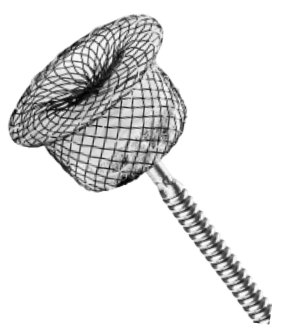
Single-hub self-expandable design helps reduce metal implants and strain on the heart .
Nitinol material offers excellent biocompatibility and shape memory.
The cylinder or cone shape ensures a close and stable fit to the wall of ductus arteriosus.
Bundling process incorporates physical kneading technology to achieve a secure press fit without the need for soldering.
PET flow-occluding fabric is engineered for the rapid cessation of blood flow upon implantation.
Models & Specifications
| WTWBFDQ-I |
04-22 |
| WTWBFDQ-II |
06-22 |
Registration Certificate No.
EU CE certification No. CE 650110
(Please consult your local dealer for details)
Information for Patient
Please read the following information carefully. If you have any questions or are not sure about the information provided below, ask your doctor.
You will receive an implant card that holds important information about your implant. If you need medical assistance, show your card to the doctor at your health facility.
Further information can be found in the European database on medical devices (Eudamed) by searching the Basic UDI-DI “69330523XZ0003K5” at: https://ec.europa.eu/tools/eudamed (When Eudamed is available)
Device description
The MemoPartTM PDA Occluder is used for transcatheter, percutaneous closure of congenital patent ductus arteriosus. The occluders are composed of Nitinol (40.6% - 84.5%), stainless steel (14.2% - 52.9%), PET (1.3% - 6.5%), PA (~0.1%). They do not contain medicinal substances, animal or human tissue; they are no blood products and are not radioactive.
Note: If you are allergic to nickel or have a history of metal allergies, you should ask your doctor. Your doctor will help you decide whether it is appropriate for you to get an occluder.
Information for safe use
Make sure you follow your doctor’s recommendations after the treatment. Not following your doctor’s advice may result in complications and the need for additional medical procedures.
Discuss any questions, concerns, or potential side effects with your doctor.
Note: If you experience any symptoms of shortness of breath or chest pain at any time, seek medical care immediately.
Magnetic Resonance Imaging (MRI)
An MRI scan of 1.5 and 3 Tesla is tested conditionally safe under specific settings and is possible to perform immediately after the procedure. Please tell your radiologist prior to an MRI scan that you carry an implant, and show your implant card.
A patient after being implanted with this device can be safely scanned immediately after implantation under the following conditions:
Static magnetic field of 3.0 Tesla and 1.5T
Maximum spatial gradient field is 20T/m in 3T and 40T/m in 1.5T MR system
Maximum whole-body specific absorption rate (SAR) of 2.0W/Kg for 15 minutes of scanning in Normal
Expected lifetime of the device
The MemoPartTM PDA Occluder is a permanent implant. Under normal conditions, the implanted device will remain in your body for life, unless it is required to be removed by the physician’s professional judgment.
Follow-up
It is important to schedule regular follow-up visits with your doctor. Follow-up visit will help the doctor to check your heart on a regular basis. The follow up visit should be performed at 24 hours, 1, 3, 6, and 12 months after the procedure, and can be adjusted by the doctor depending on your individual condition. Routine clinical follow-ups with a cardiologist annually thereafter are also advised.
Travelling
The device will not set-off any metal detectors alarms. If your implant causes an alarm at a security scanner, show your implant card to security staff.
Symbols on Implant Card
|
SYMBOL
|
DESCRIPTION OF SYMBOL
|
|
|
Patient Name or patient ID
|
|
|
Date of implantation
|
|
|
Name and Address of the implanting healthcare institution/provider
|
|
|
Information website for patients, where patients can obtain additional information on the implant.
|
|
|
Device name
|
|
|
Catalogue number of the implant
|
|
|
Unique device identifier of the implant
|
|
|
Serial number of the implant
|
|
|
MR Conditional, indicates that non-clinical testing has demonstrated that the implant can safely be scanned under specific MR conditions.
|
|
|
Name and Address of the manufacturer
|
In case of loss or degradation of the implant card, please contact your healthcare professional or the healthcare institution where your procedure took place to obtain information about a replacement. In line with data protection and patient confidentiality laws, SHSMA will not collect any information about patients or procedures where our devices are used.
Document No: IFP-PDA-001 Rev.01/2023.12.20











 沪公网安备31011702008238号沪ICP备2021017431号
沪公网安备31011702008238号沪ICP备2021017431号







