CHD Treatment Solutions

VSD Occluder
(Double-hub)
Description
For transcatheter closure of congenital ventricular septal defects.
Features
Double-hub self-expandable design is employed for secure and firm clamping.
Nitinol material offers excellent biocompatibility and shape memory.
Bundling process incorporates physical kneading technology to achieve a secure press fit without the need for soldering.
PET flow-occluding fabric is engineered for the rapid cessation of blood flow upon implantation.
Models & Specifications
| SQFDQ-I a | 04-18 |
| SQFDQ-I b | 04-18 |
| SQFDQ-I c | 04-18 |
| SQFDQ-I d | 04-18 |
| SQFDQ-II b | 04-20 |
| SQFDQ-III | 04-18 |
| SQFDQ-IV | 04-16 |
| SQDFDQ-Ⅱa | 04-20 |
Registration Certificate No.
|
Product Name
|
IFU Revision / Issue Date
|
IFU Language
|
Implant Card
|
|
MemoPart® VSD Occluder
|
Rev.07
|
EN (Click to download)
|
EN (Click to download)
|
|
Issued on: /
|

-
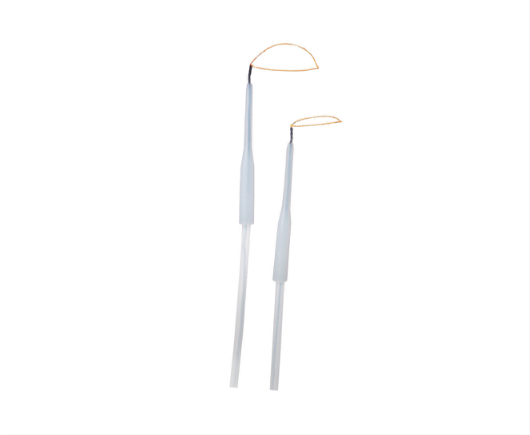
Snare
了解详情Featuring elasticity and kink resistance to allow ease of operation.
Favorable developability and improved accuracy and success rate.
-
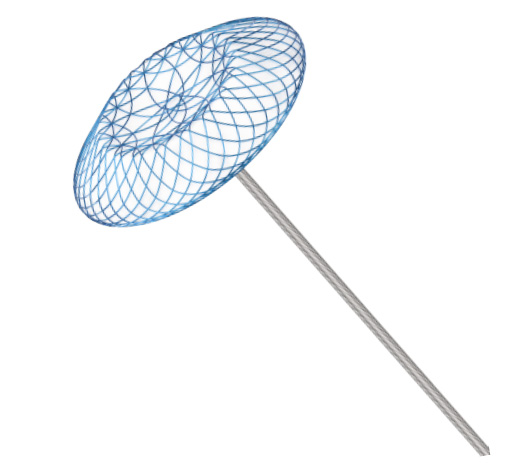
ASD Occluder (Oxide Coating)
了解详情Single-hub design: The left disc hub is replaced by the datura-shaped braided mesh for allowing more flattened discs to facilitate endothelialization.
The gaps among the disc wires preserve a pathway for future transseptal treatments.
With plasma treatment process, a dense and uniform oxide layer is formed on the surface of wire to minimize the release of nickel ions.

Address:F/5, Building 41, 258 Xinzhuan Road, Songjiang District, Shanghai, PRC
Tel:021-37015600
Zip Code:201612
-
About Us
-
Medical Solutions
-
Talent Planning and Management
-
Investor Relations
-
Media Center
-
Contact Us
Copyright © 2021, Scientech Medical. All rights reserved. 沪公网安备31011702008238号沪ICP备2021017431号
沪公网安备31011702008238号沪ICP备2021017431号






