
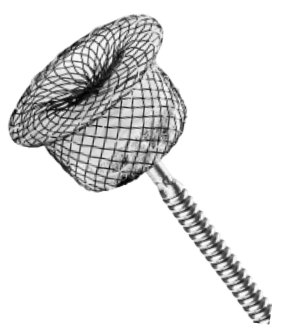
PDA Occluder (Single-hub)
Description
For transcatheter, percutaneous closure of congenital patent ductus arteriosus (PDA).
Features
Single-hub self-expandable design helps reduce metal implants and strain on the heart .
Nitinol material offers excellent biocompatibility and shape memory.
The cylinder or cone shape ensures a close and stable fit to the wall of ductus arteriosus.
Bundling process incorporates physical kneading technology to achieve a secure press fit without the need for soldering r.
PET flow-occluding fabric is engineered for the rapid cessation of blood flow upon implantation.
Models & Specifications
|
WTWBFDQ-I |
04-22 |
|
WTWBFDQ-II |
06-22 |
Registration Certificate No.
EU CE certification No. MDR 775042
(Please consult your local dealer for details)
|
Product Name
|
Safety and Performance Information
|
Information for Patient
|
|
MemoPart® PDA Occluder (Single-hub)
|
Copyright © 2021, Scientech Medical. All rights reserved. 沪公网安备31011702008238号沪ICP备2021017431号
沪公网安备31011702008238号沪ICP备2021017431号







