Good News: MemoCarna ASD Occluder III (Oxide-coating) Won National Class-Three Registration Certificate for Medical Apparatus
On May 12, 2021, the 'Oxide-coating single-rivet arterial catheter occluder' (trade name: MemoCarna oxide-coating PDA occluder) independently developed by Shanghai Shape Memory Alloy Material Co., Ltd. (hereinafter referred to as SHSMA) officially obtained class-three registration certificate for medical apparatus (State Machinery Registration Standard 20113130335) from the National Medical Products Administration, marking clinical application of another blockbuster new product in this series after launching of the oxide-coating single-rivet occluder.
In Pursuit of Product Quality With Initial Intention in Mind
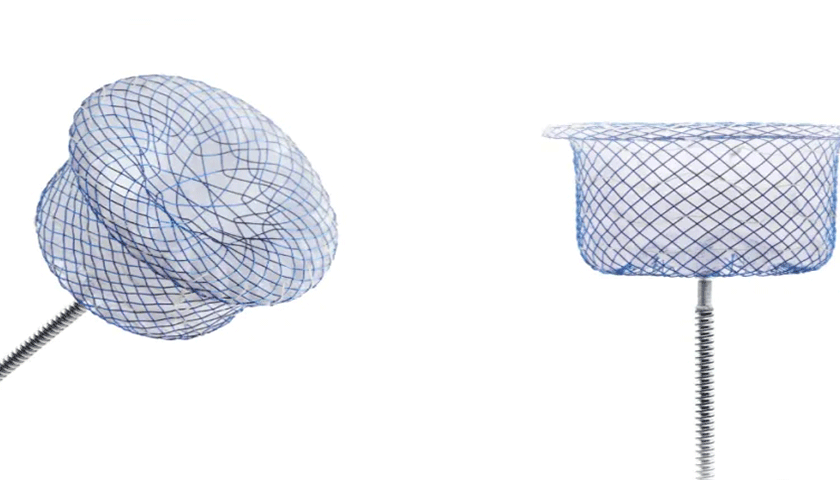
The MemoCarna oxide-coating PDA occluder is composed of nickel-titanium alloy stent and polyester fiber membrane. When compared with traditional occluder, two major upgrades of this product are single-riveting design and oxide-coating surface treatment process. It also applies cylindrical and conical design of SHSMA double-rivet PDA occluder, which would effectively target at the diseased part.
MemoCarna oxide-coating single-rivet PDA occluder has a unique braised pattern on the left of PDA occluder to replace wire rivet head seen in traditional occluder so that disk surface can be flatter and more prone to endothelialize after release. Moreover, the single-rivet design can lower the impact on blood flow and reduce incidence of postoperative complications. After oxide treatment of plasma surface, an even and dense oxide coating is formed on the surface of the nickel-titanium alloy wire, which would effectively bring down precipitation of nickel ions and maximize safety of patients.
Clinical trial of MemoCarna oxide-coating single-rivet PDA occluder’s success rate of occlusion was 100% after 6 months of the surgery. Results of clinical trial show that MemoCarna oxide-coating single-rivet PDA occluder has a high technical success rate, shows sound safety, and can be widely applied in clinical practice.
Innovation iteration adds impetus to development of the industry
MemoCarna oxide-coating single-rivet occluder series is an innovative domestic concentric occluder with independent intellectual property rights launched by SHSMA on ground of superb performance of the first generation of occluders.
In May 2020, oxide-coating single-rivet ASD occluder was granted approval for being launched to the market as the first new product in this series. After one year of clinical application after it was launched to the market, it has been recognized by clinicians for its superb performance. One year after that, the oxide-coating single-rivet series added new products to the list, which would inject new vitality to the closure industry for inborn heart disease.
A company specialized in development and production of cardiovascular interventional closure SHSMA has been deeply rooted in this field for more than two decades. Launching of a new generation of oxide-coating occluder can be said as the result of the company's continuous input in research, development and and optimization of innovative results of its existing assembly lines. In the future, the company is sure to launch superb high-end medical equipment with more professional attitude and to roundly boost development of cardiovascular health course by adhering to the corporate tenet of caring for life with science and technology.
-

Exhilarating news: ScienTech Medical’s MemoSorb® Biodegradable Patent Foramen Ovale (PFO) Occluder obtained NMPA approval
2023-09-17 -

ScienTech Medical reveals impressive one-year follow-up results of ScienCrown® Valve System for TAVR clinical study at GW-ICC2023
2023-09-13 -

Good news: Disposable Introducer Sheath obtained Class II Medical Device Registration certificate
2023-06-28
Copyright © 2021, Scientech Medical. All rights reserved. 沪公网安备31011702008238号沪ICP备2021017431号
沪公网安备31011702008238号沪ICP备2021017431号







